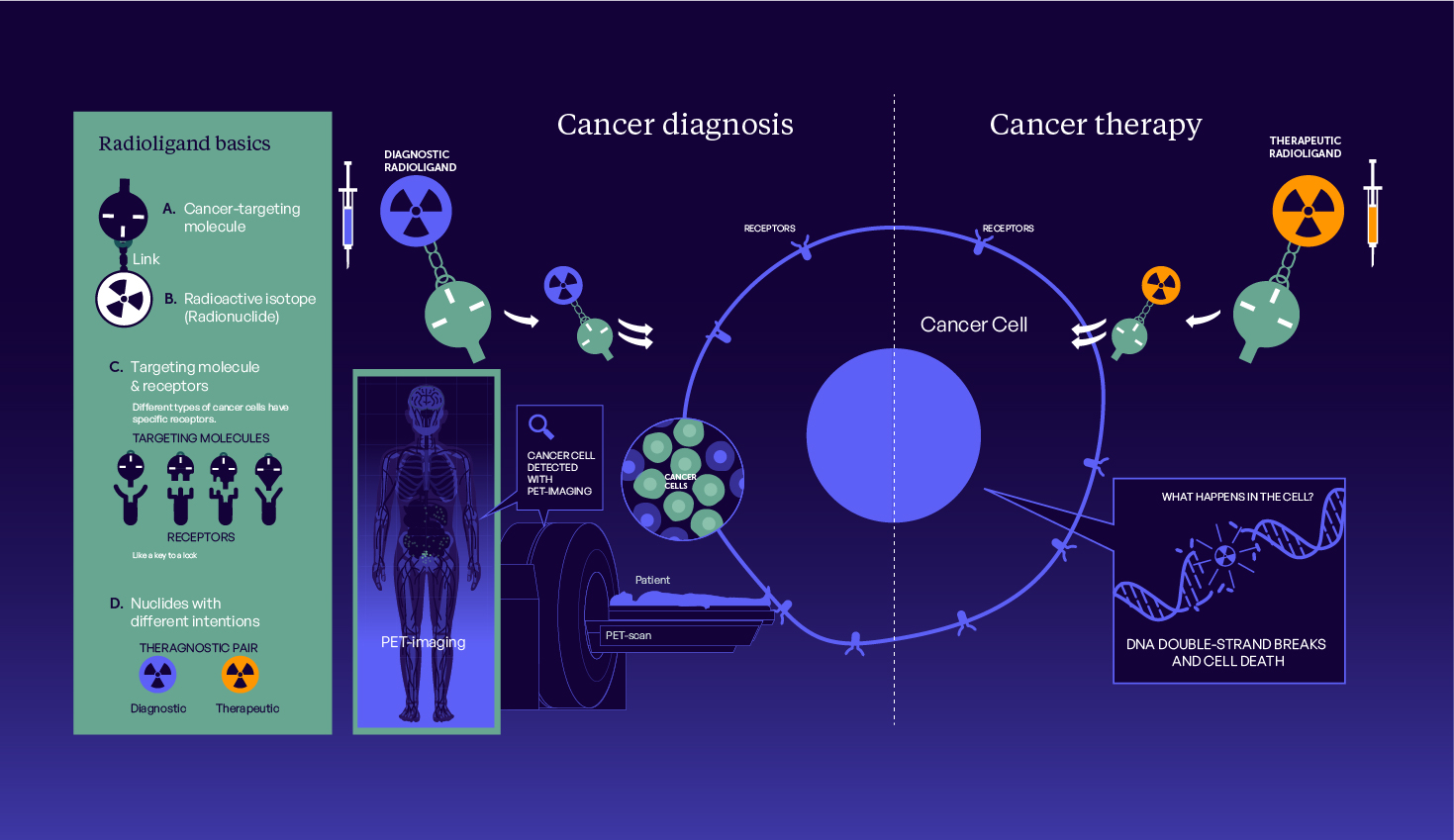
RPO Group is a radiotheranostic CRO located in the heart of Western Europe, the global epicentre of radiomolecular precision oncology. This collaborative alliance of experts including Prof Dr Richard P Baum, Prof Dr Andreas Tuerler, and Dr Hugo M L Jansen unites their respective specialities into a flexible, tailor-made approach.